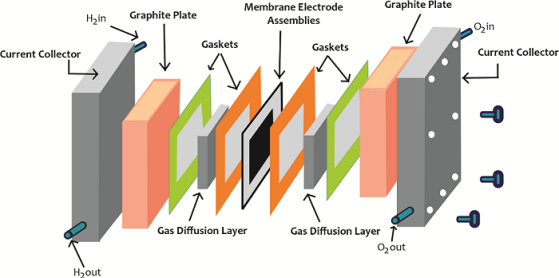
CATALYST
Catalytic reactions occur in a few basic steps. First, the reactants adsorb onto the surface of the catalyst. Then, the reaction occurs. Finally, the products desorb from the surface.

Standard Sizes
In general, a catalyst is “good” when the adsorption is favourable (or else the reaction won’t happen very fast), but the desorption is also favourable (or else the surface will quickly become cluttered, slowing the reaction). Since adsorption and desorption depend on the same property of the catalyst – adsorption energy – the quality of a given catalyst falls on a “volcano plot” for any given reaction. For both the anode and the cathode reactions in a fuel cell, platinum is a good catalyst because it falls near the peak of the volcano plot. That is, it has a good balance between favourable adsorption and favourable desorption for hydrogen, oxygen, and water.
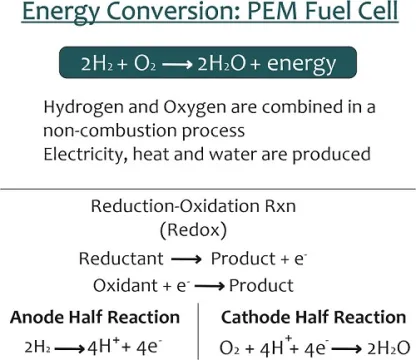
Electrochemical devices need catalyst in order to function and deliver high performance. In electrochemical device, an oxidation reaction needs to couple with a reduction reaction. The hydrogen oxidation reaction (HOR), along with the hydrogen evolution reaction (HER) is amongst the most studied reactions of modern science due to its simple reaction of one hydrogen molecule going to two protons, while releasing two electrons. Examples of such reactions are hydrogen oxidation reaction (HOR), hydrogen evolution reaction (HER), oxygen reduction reaction (ORR), water oxidation (which is also called water electrolysis), etc. Catalyst materials reduce the activation energy of reactions and hence enable manufacturing of highly efficient electrochemical devices that achieves wonders.